The Influenza SARS-CoV-2 Multiplex ValuPanel™ Reagents are separately delivered probes and primers for RT-PCR detection of the genetic sequences of SARS-CoV-2, influenza A, and influenza B viruses.
For Research Use Only. Not for use in diagnostic procedures. This product may not be used under the U.S. Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA) of the Center for Disease Control and Prevention (CDC) Influenza SARS-CoV-2 (Flu SC2) Multiplex Assay. The oligonucleotide sequences were designed by the CDC.
Key benefits
- Fast delivery: Product in stock ships same or next business day
- Gold standard quencher: From the trusted inventor and original source of Black Hole Quencher™ (BHQ™) technology referenced in 7 of 8 WHO protocols
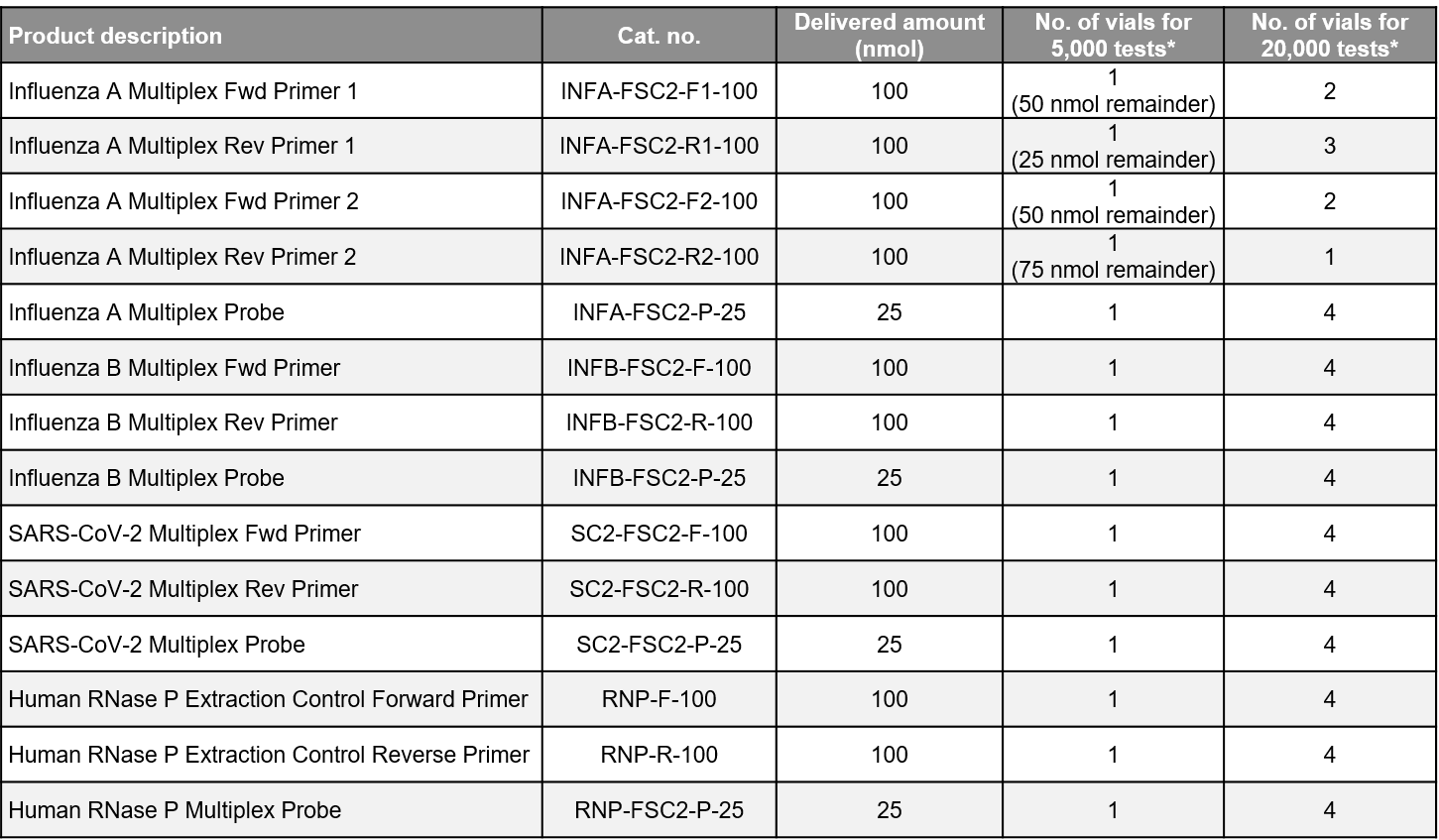
- Quality manufacture: All probes and primers are HPLC purified and are manufactured and shipped from a facility entirely separate from positive control production.

| Item | Sequence |
|---|---|
| Influenza A Multiplex Fwd Primer 1 | CAA GAC CAA TCY TGT CAC CTC TGA C |
| Influenza A Multiplex Rev Primer 1 | GCA TTY TGG ACA AAV CGT CTA CG |
| Influenza A Multiplex Fwd Primer 2 | CAA GAC CAA TYC TGT CAC CTY TGA C |
| Influenza A Multiplex Rev Primer 2 | GCA TTT TGG ATA AAG CGT CTA CG |
| Influenza A Multiplex Probe | FAM-TGC AGT CCT [Nova] CGC TCA CTG GGC ACG-BHQ-1 |
| Influenza B Multiplex Fwd Primer | TCC TCA AYT CAC TCT TCG AGC G |
| Influenza B Multiplex Rev Primer | CGG TGC TCT TGA CCA AAT TGG |
| Influenza B Multiplex Probe | CIV-550-CCA ATT CGA [BHQ-1] GCA GCT GAA ACT GCG GTG-Spacer C3 |
| SARS-CoV-2 Multiplex Fwd Primer | CTG CAG ATT TGG ATG ATT TCT CC |
| SARS-CoV-2 Multiplex Rev Primer | CCT TGT GTG GTC TGC ATG AGT TTA G |
| SARS-CoV-2 Multiplex Probe | CAL Fluor Red 610-ATT GCA ACA [Nova] ATC CAT GAG CAG TGC TGA CTC-BHQ-2 |
| Human RNase P Extraction Control Forward Primer | AGA TTT GGA CCT GCG AGC G |
| Human RNase P Extraction Control Reverse Primer | GAG CGG CTG TCT CCA CAA GT |
| Human RNase P Multiplex Probe | Quasar 670-TTC TGA CCT [Nova] GAA GGC TCT GCG CG-BHQ-2 |